Dairy Chemical Quality Testing Experiment
| ✓ Paper Type: Free Assignment | ✓ Study Level: University / Undergraduate |
| ✓ Wordcount: 6292 words | ✓ Published: 06 Jun 2019 |
Dairy Chemistry Practical Write Ups Chemical Quality Testing Essay
Table of Contents
- 1. Volumetric analysis. Acetic acid content of vinegar
- 1.2 Introduction:
- 1.3 Aim
- 1.4 Health and safety
- 1.5 Materials and Methods:
- 1.5.1 Task 1: Standard solution making
- 1.5.2 Task 2: Titration
- 1.5.3 Task 3: Percentage determination of acetic acid in vinegar
- 1.6 Results and calculations
- 1.7 Discussion
- 1.8 References
- 2. Qualitative detection of carbohydrates using food samples.
- 2.1 Introduction
- 2.2 Aim
- 2.3 Health and safety
- 2.4 Materials and Methods:
- 2.5 Results:
- 2.6 Discussion:
- 2.7 References
- 3. Determination of fat in Wensleydale cheese using the Gerber Method.
- 3.1 Introduction
- 3.2 Aim
- 3.3 Health and safety
- 3.4 Materials and Methods:
- 3.5 Results of the Gerber method
- 3.6 Discussion
- 3.7 References
- 4. Determination of crude protein in milk by the Kjeldahl method
- 4.1 Introduction
- 4.2 Aim
- 4.3 Health and safety
- 4.4 Materials and Methods:
- 4.5 Calculations
- 4.6 Discussion
- 4.7 References
- 5. Chemical Quality Testing in Modern Dairy Business
- References
1. Volumetric analysis. Acetic acid content of vinegar
1.2 Introduction
Volumetric Analysis is a method in which the amount of a substance is determined by measuring the volume of a second substance that combines with the first in known proportions. Usually, it is used to determine the unknown concentration of known reactant. In this particular experiment, the Sodium Hydroxide (NaOH) molar concentration was known (0.1 M). In chemistry, molarity is a concentration unit, defined to be the number of moles of solute divided by the number of litres of solution (Helmenstine, 2016). In this volumetric analysis, Hydrochloric Acid (HCI) concentrations have not been given and were meant to be defined.
Volumetric analysis is often referred to as titration. The procedure of titration is used for determining the amount of acid (or base) in a solution by determining the volume of base (or acid) of a known concentration that will ultimately react to it. Several inorganic acids and bases and hundreds of organic compounds are sufficiently determined using this method(Csuros,1998).Vinegar is a common household item containing acetic acid as well as some other chemicals. To determine the molarity of acetic acid in the vinegar sample it is necessary to calculate moles of NaOH which is required to neutralise the acetic acid in vinegar(SchoolWorkHelper, 2017).Legally the amount of acetic acid in the vinegar has to have minimum 4% of acetic acid (Fda.gov, 2017).
This analysis involves three primary processes: determining the concentration of HCl solution, determining the percentage of acetic acid in vinegar and the titrations performed. To determine the molarity of acetic acid in vinegar sample it is necessary to calculate moles of NaOH which is required to neutralise the acetic acid in vinegar.
1.3 Aim
The aim was to determine the molarity of the 0.1 M hydrochloric acid solution, also to determine the level of acetic acid in vinegar sample during titration process.
1.4 Health and safety
- It is the responsibility of everybody who is working in the laboratory to take reasonable care of themselves and those working around them.
- Personal Protective Equipment (PPE) should be worn all the time in the laboratory such as:
- Safety glasses or goggles.
- Provided coat
- Hear net
- Gloves if needed.
Use of NaOH
Solid sodium hydroxide is corrosive, hygroscopic and caustic, therefore should not come into contact with exposed body parts, or metallic surfaces of the weighing balance. Though fairly corrosive when dilute, NaOH is a mild irritant that should be handled with care. In case aqueous NaOH spills on naked skin surfaces, proper rinsing with flowing water should be administered, while any spills on the working benches should be cleaned instantly. Preparation of aqueous NaOH solutions from its solid state should only be done by adding solid NaOH into the water in the conical flasks, and not the reverse.
Use of HCl
Though dilute, HCl is a highly corrosive acid that should be kept away from the human skin, metal surfaces and the working benches. In the case of any spills, the acid should be cleaned with plenty of water.
Use of Vinegar
Vinegar is an HC2h4O2 (acetic acid) solution, and so likely to corrode metal surfaces. At its native state, it is a mild irritant and should be kept away from coming into contact with human skin and anybody openings. Vinegar should be used in a place with free air circulation.
1.5 Materials and Methods:
1.5.1 Task 1: Standard solution making
- 2 g of solid sodium hydroxide (NaOH)was weighed out using laboratory scales and weight boat.
- 100 g of water measured using measuring cylinder
- Required amount of NaOH was transferred into labelled beaker
- Water added to beaker with NaOH and stirred until NaOH completely dissolved
- Solution was transferred into 500 ml volumetric flask, and water added up to the mark
- Solution stirred through
1.5.2 Task 2: Titration
- 250 ml conical flask was rinsed with distilled water
- 25 ml of 0.1 M hydrochloric acid (HCl) solution was pipetted and added to conical flask
- 2-3 Drops of indicator phenolphthalein then added in to solution
- The burette was properly rinsed out with 0.1 M NaOH
- 50 ml burette filled with NaOH (0.1 M)
- NaOH was then used to titrate into the conical flask to reach the end point (Colour change).
- Colour appeared light pink.
- Readings were taken at the end point
- Repeated two –three times until three readings with no more than 0.1 ml were achieved
1.5.3 Task 3: Percentage determination of acetic acid in vinegar
- 10 ml of vinegar was pipetted into 100ml volumetric flask
- Water added until the mark
- 3 drops of phenolphthalein were added into vinegar solution
- A 50ml burette was filled with 0.1 M NaOH solution
- Titration started until the colour of resultant solution changed from colourless to pale pink.
- The final reading of burette was then recorded
- The procedure was repeated in order to achieve second reading, aiming for the two be within 0.2 ml of each other.
1.6 Results and calculations
Table 1: Amount of NaOH 0.1 M required for titrating 25 ml HCl solution of 0.1M
| 1 | 2 | 3 | |
| Initial reading | 0 ml | 0 ml | 0ml |
| Final reading | 30.1 ml | 30 ml | 30.1 ml |
| Volume used | 30.1 ml | 30 ml | 30.1ml |
Three titrations appeared within 0.1 ml of each other as required. The following calculations were done to determine the molarity of the HCl:
 Molarity of HCl = Volume of NaOH x 0.1
Molarity of HCl = Volume of NaOH x 0.1
25
Using the formula provided results appeared: Molarity of HCl=0.1202M
0.1 M of NaOH should neutralise0.1 M of HCl.
Table 2: Amount of NaOH required to titrate 25 ml of vinegar
| 1 | 2 | 3 | |
| Initial reading | 0 ml | 0 ml | 0 ml |
| Final reading | 25.2 ml | 25.2 ml | 25.3 ml |
| Volume used | 25.2 ml | 25.2 ml | 25.3 ml |
Titration was carried out until two readings within 0.2 ml were achieved, as per practical handout requirements:
25.2 x 25.23= 25.25 ml2
For the calculation of percentage of acetic acid, formula used below:
Percentage (w/v) acetic acid = volume of 0.1 M NaOH x 0.24
Which then appears that percentage of acetic acid in this particular case equals to 6.06%.
1.7 Discussion
Experiment one
The amounts of HCl were meant to be 0.1 M, and results appeared as 0.12 M, which is a little high. Therefore, there have been some errors in the procedure which may be influencing the results. The following discussion will explain possible errors which may appear in the process such as intrinsic error in the method, the end point is not identical every time, as the colour changes are not instant. The equivalence point is not always identical. There was possibility of using an excessive amount of NaOH this may happen due to the fact that titrant colour of the end point wasn’t identified correctly. Another possible reason for this particular mismatch could have been human error, during the amount of liquid filled up into the burette. Another possible reason could have been misreading the volume, for example, a parallax problem (when someone reads the volume looking at an angle), or error in counting unmarked graduation marks. When reading the volume on the burette scale it is not uncommon to read both upper and lower value in different lighting conditions, which can make a difference.
Experiment two
Expecting acetic acid in vinegar has to be at least 4% as that’s the legal requirement; the results appeared in the titration of vinegar where 6.06% which is a good result (Fda.gov, 2017). However, also there could be some errors in the true value as the same set-up was used as for experiment one. Vinegar that has a minimum of 4% acetic acid and a maximum of 8% conforms to legislation and can be legally sold in the UK. So if the value was correct at 6.06%, potentially there could be an opportunity for the company to save money by reducing the acetic acid content closer to the 4% minimum.This could be achieved by adding water, therefore, reducing the amount of acid used. Acetic acid is obtained from first producing alcohol from malt and then using acetic acid bacteria to produce acetic acid. The traditional method is done in a barrel half filled with alcohol and takes a long time. The quicker way is to pump oxygen through the liquid. As the most expensive component in the vinegar is the acid, reducing the acid level would save a lot of money, as long as this did not affect the quality characteristics of the product, such as colour, flavour and aroma.
1.8 References
Csuros, M. (1998). Environmental sampling and analysis. 1st ed. Boca Raton: Springer.
Fda.gov. (2017). CPG Sec. 525.825 Vinegar, Definitions – Adulteration with Vinegar Eels. [online] Available at: https://www.fda.gov/iceci/compliancemanuals/compliancepolicyguidancemanual/ucm074471.htm [Accessed 25 Apr. 2017].
Helmenstine, A. (2016). Here’s What Molarity Means in Chemistry (Definition and Examples). [online] ThoughtCo. Available at: https://www.thoughtco.com/molarity-definition-in-chemistry-606376 [Accessed 25 Apr. 2017].
SchoolWorkHelper. (2017). Titration of Vinegar Lab Answers. [online] Available at: https://schoolworkhelper.net/titration-of-vinegar-lab-answers/ [Accessed 25 Apr. 2017].
2. Qualitative detection of carbohydrates using food samples.
2.1 Introduction
The qualitative and quantitative analysis is used to determine the composition of foods, beverages and their ingredient’s (Nielsen, 2003). Qualitative analysis in this particular test was used to determine the presence of carbohydrates in food samples. Carbohydrates in food can include anything from the simple monosaccharide – glucose to the very complex polysaccharide’s (Eliasson, 2006). Carbohydrates can be reducing or non-reducing sugars; they can be detected through the use of Benedict test, Fehling’s test and Molisch test. Benedict is a test which determines the presence of reducing sugars because of the way it has been prepared and carried out. It can be determined by colour changes – blue would stand as a control point, brick red would mean positive result (reducing sugars) (Anon, 2017). Fehling’s is another test to determine reducing sugars in food samples. In this test the presence of aldehydes but not ketones is detected by reduction of the deep blue solution of copper (II) to a red precipitate of insoluble copper oxide (Anon, 2017). Molisch test is used to determine carbohydrates in general. This is based on the dehydration of the carbohydrate by sulfuric acid or hydrochloric acid to produce an aldehyde, which condenses with two molecules of phenol resulting in a red- or purple-coloured food samples which will indicate positive results (Guiwa, 2017)
2.2 Aim
The aim is to detect of carbohydrates and reducing sugars in food samples provided.
2.3 Health and safety
- It is the responsibility of everybody who is working in the laboratory to take reasonable care of themselves and those working around them.
- Personal Protective Equipment (PPE) should be worn all the time in the laboratory such as:
- Safety glasses or goggles.
- Provided coat
- Hear net
- Gloves if needed.
2.4 Materials and Methods:
Solid food was ground up in pestle and mortar before testing. There were six different types of food samples placed in clean test tubes and this step been followed by adding 6 ml of water into test tubes. Tubes have been shacked in order to dissolve as much as possible carbohydrates. Then it has been divided into three aliquots for following tests.
1. For Benedict test, there were added 2 ml of Benedict reagent into samples and it has been placed in boiling water bath for five minutes. After five minutes it has been taken out and colour changes have been recorder.
1. The table below: Colour changes meaning:
| Observation (Final colour change) | Interpretation |
| No colour change ( Blue) | No reducing sugars present |
| Green | Trace amounts of reducing sugar present |
| Yellow | Low amounts or reducing sugars present |
| Orange | Moderate amount of reducing sugars present |
| Brick-red | Large amounts of reducing sugars present |
(Biology Notes for IGCSE 2014)
2. For Fehling’s solution (equal volume of Fehling’s A and B solutions) 2 ml were added into test tubes and it has been placed into boiling water bath for three minutes. After required time samples had been taken out of water bath and results has been recorded. If solution changes colour from blue and forms a red or green precipitate then it means this test is a positive result.
3. For Molisch test 5 drops of reagent has been added to the test tubes and shaked well. Then 1 ml of concentrated sulphuric acid carefully added. And results appeared has been recorded If results has a purple ring at the junction of the two layers, this counts as positive result.
2.5 Results:
1. The table below provides with results appeared during testing:
| Practical used | Biscuit | Gelatine | Milk | White
flower |
Whole
flower |
Unknown |
| Benedict’s | Negative | Negative | Positive | Negative | Negative | Negative |
| Fehling’s | Negative | Negative | Positive | Negative | Negative | Negative |
| Molisch | Positive | Negative | Positive | Positive | Positive | Positive |
2.6 Discussion:
Molisch test is different from the other tests because it is used as a general test to detect any type of carbohydrates. The Molisch test will also detect glycolipids and glycoproteins whether in combined or free form. The solution contained strong acids which hydrolysed carbohydrates to monosaccharides by use of beta-naphthol. In the food samples of the gelatine, the test was negative although gelatine is believed to contain glucose (or possibly mannose or galactose) combined in the form of a polysaccharide (Wood, 2016), but the different test should be applied to find carbohydrates in gelatine. In the samples of biscuit, milk, whole flour, white flour and unknown samples, the result was positive due to the formation of a deep violet. The colour arose from the formation of unstable beta-naphthol condensation products (Montero, C, 2004) Results indicated that there were different compositions of carbohydrates founded in sample tests.
Benedict’s solution determines the presence of reducing sugars. For the food samples we used, the results were negative in all the food samples apart from milk. Milk colour changed from white to green/yellow precipitate and finally brick red after heating, hence indicating the presence of reducing sugars. Milk was able to exchange hydrogen electrons up to a certain degree and reduced cupric ions (Cu2+) to cuprous (Cu+). (Stanley R, 2009) Lactose is found in milk and it is a disaccharide, which means it’s made up of two monosaccharide units. Specifically, the monosaccharides in lactose are glucose and galactose (Hendrickson and Hendrickson, 2016). So result for Benedict was achieved as expected.
In Fehling’s test, Fehling reagent is blue in colour. It differentiates reducing sugars (those with open chains) from aldehydes. It was negative in the other food solutions but tested positive in milk since a brick red precipitate was formed. This shows that milk was able to reduce the sugars that were present hence cuprous oxide was formed. Milk is lactose hence there were positive results. The other food solutions contained starch or cellulose hence turned negative, indicating they were polysaccharides which formed many chemical rings (Albalasmeh, Berhe and Ghezzehei, 2013)
2.7 References
Albalasmeh, A., Berhe, A. and Ghezzehei, T. (2013). A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydrate Polymers, 97(2).
Anon, (2017). Reducing sugars. [online] Available at: https://fenix.tecnico.ulisboa.pt/downloadFile/3779571247498/Testes%20de%20a%C3%A7ucares-alunos.pdf [Accessed 27 Apr. 2017].
Biology Notes for IGCSE 2014. (2017). Food test 2 – Benedict’s test for Reducing Sugars. [online] Available at: http://biology-igcse.weebly.com/food-test-2—benedicts-test-for-reducing-sugars.html [Accessed 27 Apr. 2017].
Eliasson, A. (2006). Carbohydrates in Food, Second Edition. 1st ed. Boca Raton Forida: Taytor and frances group.
Guiwa, L. (2017). molisch | Acid. [online] Scribd. Available at: https://www.scribd.com/document/47600264/molisch [Accessed 27 Apr. 2017].
Hendrickson, K. and Hendrickson, K. (2016). What Type of Carbohydrate Is Lactose?. [online] LIVESTRONG.COM. Available at: http://www.livestrong.com/article/408075-what-type-of-carbohydrate-is-lactose/ [Accessed 28 Apr. 2017].
Montero, C, M. (2004). Analysis of low molecular weight carbohydrates in food and beverages: a review. Chromatographia, (59.1), pp.15-30.
Nielsen, S. (2003). Instructor’s manual for Food analysis, third edition. New York: Kluwer Academic/Plenum Publishers.
Stanley R, B. (2009). A reagent for the detection of reducing sugars. Journal of Biological Chemistry, 5.5, pp.485-487.
Wood, H. (2016). The Carbohydrate of Gelatin: The Journal of Photographic Science: Vol 6, No 3. [online] Tandfonline.com. Available at: http://www.tandfonline.com/doi/abs/10.1080/00223638.1958.11736630?journalCode=yims19 [Accessed 28 Apr. 2017].
3. Determination of fat in Wensleydale cheese using the Gerber Method.
3.1 Introduction
Fats are the substances that are soluble in organic solvents such as ether, hexane, and chloroform but are insoluble in water (Akoh and Min, 2002). Milk fat and milk proteins (casein) are primary constituents of cheese. Milk fat is the richest energy component of milk. It contains a high proportion of saturated fatty acids, with one-third of the total content consisting of lauric acid, meristic acid, and palmitic acid (1). In the milk of most mammals, 97–98% of the total fat content is composed of triglycerides (Mlcek et al., 2016). Fat and protein ratio has a significant impact on the finished product, as it affects firmness, mouthfeel, texture and the flavour qualities of cheese (Fox, 1997). There are fats which consist of triacylglycerol’s, diacylglycerols, monoacylglycerols and several fatty acids (Scientificpsychic.com, n.d.). To specify the amount of fat percentage in cheese, there are standard methods which are based on either weight or volumetric determination. In this particular case, Gerber method has been used to analyse fat content in Wensleydale cheese, this type of cheese usually contains 31.8% of fat (McCance, 2015). Gerber method is a volumetric method in which fat is separated from cheese by centrifugal force. Sulphuric acid is used to dissolve the protein that forms the membrane around the fat (fat globules), and amyl alcohol is added to improve the separation of fat from other solids (Pearson, Kirk and Sawyer, 1991).
3.2 Aim
The aim of this test is to define the fat content of the Wensleydale cheese provided.
3.3 Health and safety
- It is the responsibility of everybody who is working in the laboratory to take reasonable care of themselves and those working around them.
- Personal Protective Equipment (PPE) should be worn all the time in the laboratory such as:
- Safety glasses or goggles.
- Provided coat
- Hair net
- Gloves.
- Extra care is necessary dealing with sulphuric acid as it highly corrosive.
- All safety equipment has to be used while mixing Gerber butyrometer such as rack with Perspex glass towards you while mixing sample.
- Clean as you go policy
3.4 Materials and Methods:
- Clean Butyrometer was placed into the rack
- 10ml of sulphuric acid carefully dispensed into Butyrometer with precaution taken to make sure that neck wasn’t wet with acid.
- 3gr of cheese weighed and placed into blue tissue and filled into Butyrometer
- 1 ml of amyl measured and dispensed into butyrometer
- Butyrometer filed with distilled water until the shoulder below the neck.
- Lock stopper firmly placed into the neck of Butyrometer, by using the key.
- Lid of the shaking stand placed for safety
- Shacked the butyrometer in the shaker stand and then inverted until everything was mixed and dissolved.
- Butyrometer placed into the centrifugal unit and been balanced out.
- Centrifugal unit been set up for 1100rpm for at least 4 minutes and started.
- Sample has been placed stopper downwards into controlled 65ºC +/- 2ºC water bath for 3 minutes.
- The butterfat percentage was read from the lowest point of the meniscus of the interface of the acid-fat to the 0-mark of the scale
- Butyrometer placed into water bath for following 3 minutes
- The fat percentage was reread again.
3.5 Results of the Gerber method
| Cheese type | Gerber result |
| Wensleydale first result | 31.1% |
| Wensleydale second result | 31.1% |
3.6 Discussion
From this experiment, the method was successful in analysing the content of fat in the Wensleydale brand of cheese used. The method is accurate as the results had 0.1% margin of error apart, the first trial gave 31.1%, and on repeat, the fat content was 31.1%, showing the method is accurate when the procedures are followed well. Although the result was not entirely correct quantifying them with 31.85% of fat in a sample which was expected to receive (McCance, 2015), this results indicates that there was the margin of 0.7% difference between expected result and those which appeared. This potentially may mean that there were some errors during the procedure, which wasn’t fully followed. Some of the potential error could have been: a human error like misreading butyrometer, failure to mix the acid and the cheese evenly, incorrect sample or reagents weight/measure, etc. Although test had small margin this test should not be accepted, and preference of redoing it should be taken into consideration because of the legal requirement of labelling such as European Food Information to Consumers Regulation No 1169/2011 (Food.gov.uk, 2011).
3.7 References
- Akoh, C. and Min, D. (2002). Food lipids. New York: M. Dekker.
- Food.gov.uk. (2011). European Food Information to Consumers Regulation No 1169/2011 (FIC) | Food Standards Agency. [online] Available at: https://www.food.gov.uk/enforcement/regulation/fir/labelling [Accessed 30 Apr. 2017].
- Fox, P. (1997). Advanced Dairy Chemistry Volume 3. 1st ed. Boston, MA: Springer US.
- McCance (2015). Composition of foods integrated dataset (CoFID) – GOV.UK. [online] Gov.uk. Available at: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid [Accessed 30 Apr. 2017].
- Mlcek, J., Dvorak, L., Sustova, K. and Szwedziak, K. (2016). Accuracy of the FT-NIR Method in Evaluating the Fat Content of Milk Using Calibration Models Developed for the Reference Methods According to Röse-Gottlieb and Gerber. Journal of AOAC International, 99(5), pp.1305-1309.
- Pearson, D., Kirk, R. and Sawyer, R. (1991). Pearson’s Composition and analysis of foods. Harlow: Longman Scientific and Technical.
- Scientificpsychic.com. (n.d.). Fats, Oils, Fatty Acids, Triglycerides – Chemical Structure (Page 2 of 3). [online] Available at: http://www.scientificpsychic.com/fitness/fattyacids1.html [Accessed 30 Apr. 2017].
4. Determination of crude protein in milk by the Kjeldahl method
4.1 Introduction
Nitrogen, contained in the raw materials and food products, are in protein and non-protein compounds. Protein is a source of amino acids, and it is one of the major nutritional components. Proteins are large a group of organic compounds found in all living things (Ng’andwe, 2017). Milk total proteins are composed of casein, whey proteins and non-protein nitrogen (Ruska, 2014). Milk proteins are very valued as they are the main component used to produce curd and cheese. Crude protein basically is total protein, which estimated by measuring the total nitrogen content of milk. Nitrogen is multiplied by 6.38 to express the results on a protein equivalent basis. The total amount of nitrogen in milk, however, comes from both protein and non-protein sources. True protein reflects only the nitrogen associated with protein and does not include the nitrogen from non-protein sources (Lynch and Lynch, n.d.). The Kjeldahl method is based on wet combustion of the sample by heating with concentrated sulphuric acid in the presence of a metallic or other catalyst to effect the reduction of organic nitrogen in the sample to ammonia, which is retained in solution as ammonium sulphate. The digest, having been made alkaline, is distilled or steam-distilled to release the ammonia which is trapped and titrated (Pearson, Kirk and Sawyer, 1991).
4.2 Aim
To determine the percentage of protein content in milk sample using the Kjeldahl method.
4.3 Health and safety
General Safety Awareness:
- It is the responsibility of everybody who is working in the laboratory to take reasonable care of themselves and those working around them.
- Personal Protective Equipment (PPE) should be worn all the time in the laboratory such as:
- Safety glasses or goggles.
- Provided coat
- Hair net
- Gloves if needed.
- Familiarisation with all aspects of safety before using any equipment.
- No eating, drinking, smoking in the laboratory as it is prohibited.
- Clean as you go policy
- Extra care is necessary when dealing with concentrated sulphuric acid which is used in the Kjeldahl experiment. Sulphuric acid is highly corrosive.
- Awareness’ of the high-temperature heating block
4.4 Materials and Methods:
Part 1: Digestion
- Milk (2ml) was measured and poured into a digestion tube.
- 2 Kjeldahl catalyst tablets were added to the digestion tube.
- 12ml of Nitrogen free concentrated sulphuric acid was measured and added to the digestion tube with the sample.
- Digestion block was heated to 390°.
- Sample with all the ingredients was placed into the digestion block.
- Exhaust cap placed, vacuum adjusted and sample left in the heating block until liquid appeared clear greenish blue colour.
- The digestion tube was removed from the heat block and placed to cool in test tube rack for 10 minutes.
- 75 ml of distilled water was added.
- Blank sample issued as above with the milk excluded.
A Digestion is the decomposition of nitrogen in an organic sample such as milk; it utilises the concentrated sulphuric acid solution where the sample is boiled in concentrated sulphuric acid. At the end of this step, it is appearing as ammonium sulphate solution.
Organic nitrogen + H2SO4 (NH4)2SO4 + CO2 + H2O
Part 2: Distillation
- In a separate conical flask Pipette, 25ml of boric acid + Indicator solution was placed.
- The conical flask was placed into the Vapodest 30 machine on the right side as the picture above.
- The digestion tube was placed into the left side of the machine.
- Door closed.
- Steam valve had to be opened
- 1 shot of 40% sodium hydroxide solution was dispensed.
- Distillation was continued until 100-120 ml of distillate has been collected.
- Same procedure followed for the Blank sample.
Distillation step involves the conversion of ammonium ions (NH4) into ammonia (Nh4), this is made possible by adding an excess base to the acid digestion, followed by boiling and condensation. The reaction proceeds as follows:
(NH4)2SO4 + 2NaOH 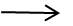 Na2SO4 + H2O + NH3
Na2SO4 + H2O + NH3
The Ammonia gas formed is collected in excess boric acid.
Part 3: Titration
- A 50ml burette was filled with hydrochloric acid (0.1m)
- The hydrochloric acid was the used to titrate into the conical flask to end point (Colour change).
- Colour appeared light pink as the picture above.
- The Same procedure was followed for the Blank sample.
- The result of titration collected for the following step.
- Same steps followed for blank sample
This method quantifies the amount of nitrogen in the milk sample. It is estimated by titration of the Ammonium Borate extracted during digestion, against Hydrochloric acid until the end point. The reaction proceeds as shown below:
2NH4H2BO3 + H2S04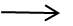 (NH4)2SO4 + 2 H3BO3
(NH4)2SO4 + 2 H3BO3
The overall reaction for this titration involves the formation of ammonium sulphate using sulphuric acid:
2 NH3 + H2SO4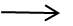 (NH4)2SO4
(NH4)2SO4
4.5 Calculations
The following formula is used to estimate the protein content in the sample being analysed
% Crude Protein = Titration figure x 0.0014 x conversion figure x100
Weight of original sample
The conversion figure for milk and dairy products is usually 6.38
In this particular titration, the titre volume (titration figure) for the milk was: 10.5
The weight of the sample was: 2 grammes
% Crude Protein = 10.5 x 0.0014 x 6.38 x100
2 gm.
Percentage of crude protein in sample: 4.68%
Blank sample titre volume result was: 1.2 ml
% Crude Protein = 1.2x 0.0014 x 6.38 x100
2 gm.
Percentage of crude protein in blank sample: 1.07%
4.6 Discussion
The protein content of milk sample used in this experiment appeared to be high as estimated by the Kjeldahl Method; the content obtained was 4.68%. This result appeared slightly higher than normal quantities from cow’s milk, this highlight the fact that the milk sample might have been collected from a specific indigenous breed of cows or another mammal with high protein content in milk. The result obtained highlight a couple more aspects which usually affects protein contest in milk including:
- Breed
- Time of the year
- Cattle average age
- Stage of lactation
Changes may occur within the milk sample: human error – such as sampling/titration error, mechanical error -such as cooling problems in raw milk tank, over agitation in the pipeline.
The accuracy and precision of the Kjeldahl method have earned it an international recognition in the estimation of the organic nitrogen (proteins) in dairy products and other foods (Lynch and Barbano, 1999). It is a standard method in which others are measured based on it.it is also used to assay nitrogen in fertilisers and soil science to estimate the nitrogen content (Amamcharla and Metzger, 2010). One challenge with it is the fact that it does not separate between the nitrogen in the proteins and the non-protein nitrogen, fraudsters has used this weakness to adulterate the milk product accessing the global market. For instance, in 2008, where a Chinese company added melamine into their milk powder to record high protein content, this method could not detect the chemical as it only quantifies the total nitrogen present (Pei et al., 2011). Also, it is challenged by the difference in the sequences of amino acids in different samples, other disadvantages of the method include, long time and procedure, high temperatures and acidic pH which can denature the proteins being analysed (Wiles PG, 1998). The method is poor in sensitivity, and modern methods for quantification of NH4 have been invented and works after the process of mineralisation and distillation has been done, they include the plasma atomic emission spectroscopy, ion exchange chromatography, capillary electrophoresis and potentiometric titrations (Kamizake et al., 2003).
4.7 References
- Amamcharla, J. and Metzger, L. (2010). Evaluation of a rapid protein analyzer for determination of protein in milk and cream. Journal of Dairy Science, 93(8), pp.3846-3857.
- Kamizake, N., Gonçalves, M., Zaia, C. and Zaia, D. (2003). Determination of total proteins in cow milk powder samples: a comparative study between the Kjeldahl method and spectrophotometric methods. Journal of Food Composition and Analysis, 16(4), pp.507-516.
- Lynch, D. and Lynch, J. (n.d.). Protein Facts. [online] Fmmaseattle.com. Available at: http://www.fmmaseattle.com/lab/FAQ.htm [Accessed 30 Apr. 2017].
- Lynch, J. and Barbano, D. (1999). Kjeldahl Nitrogen Analysis as a Reference Method for Protein Determination in Dairy Products. FOOD COMPOSITION AND ADDITIVE, 82(6), pp.1389-1390.
- Ng’andwe, C. (2017). Chemistry of proteins.
- Pearson, D., Kirk, R. and Sawyer, R. (1991). Pearson’s Composition and analysis of foods. 1st ed. Harlow: Longman Scientific and Technical, pp.15-17.
- Pei, X., Tandon, A., Alldrick, A., Giorgi, L., Huang, W. and Yang, R. (2011). The China melamine milk scandal and its implications for food safety regulation. Food Policy, 36(3), pp.412-420.
- Ruska, D. (2014). Crude Protein and Non-protein Nitrogen Content in Dairy Cow Milk. Former: Proceedings of the Latvia University of Agriculture, 32(1), pp.1-20.
- Wiles PG, e. (1998). Routine analysis of proteins by Kjeldahl and Dumas methods: review and interlaboratory study using dairy products. – PubMed – NCBI. [online] Ncbi.nlm.nih.gov. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9606925 [Accessed 30 Apr. 2017].
5. Chemical Quality Testing in Modern Dairy Business
Dairy business activities or dairy farming entails the agricultural part in the production of milk and its other products. The milk is processed on the farms or in the dairy plants to come up with dairy products which are ready for sale. Dairy businesses in developed countries such as in the UK have large dairy producing farms (Merino, Örnemark, and Toldrá, 2016). Some of the chemicals are usually categorised together into wide groups like protein, fat, mineral salts, moisture, and anhydrous lactose. All these chemicals are known to add up to 100% of total contents in dairy products. However, this analysis mostly does not contain exact measures due to some errors during the experimental operations when carrying out the required tests and some other known system errors from the equipment used in testing the components of dairy products.
Due to an increase in the usage of milk products such as whey, yogurt, and cheese by consumers, the dairy industry has come up with developed systems and products in order to meet consumer needs (Spellman, O’Cuinn, and Fitzgerald, 2005). Due to these changes, there is a need for dairy industries, consumers, and governments to check on the quality and safety of the dairy products. Some modern dairy industries have introduced various milk testing equipment to ensure that the milk is safe and the product’s quality is improved. Safe, good-quality raw milk has to be free of unwanted chemicals such as detergents and antibiotics, flavours, free of sediments and debris, abnormal odour and colour, have a low bacterial count, and a normal pH and acidity (Bylund, 1995). The quality and safety of raw milk determine the quality of the milk products as well. For this quality to be achieved, good hygiene operations should be observed in the entire dairy production chain. Inadequate skills and knowledge in hygiene matters of dairy production, uncontrolled and informal markets are known to be the main causes of difficulties among small-scale dairy producers in achieving hygienic and quality milk products. The entire handling and production processes of milk products should be provided with enough financial support to allow the improvement of dairy product quality (Butler, 2011).
Milk quality regulation and testing should be done in all stages of dairy production, whereby milk can be checked for compositional elements like protein contents and fat. Quantity should also be measured in terms of weight and volume. Drug components, chemical, and physical characteristics should also be tested. The microorganism count in the milk products is ever changing, unless the milk is sterilised or kept under low temperatures. Some of the contaminants believed impact upon the safety of raw milk are chemical, biological, or microbial. An increase in the microorganism count makes dairy products harmful for human consumption.
One of the most common microbial problems that affect the safety and quality of raw milk is mastitis, which is a well-known inflammatory condition leading to the presence of blood in the milk. The milk maybe becomes watery and discoloured; therefore mixing it with the other pure milk contaminates it. Chemical contaminants such as sterilisers and detergents are regarded as potential dangers and the failure to keep the production reservoirs and equipment free of these contaminants is often due to mismanagement and unhygienic dairy farm production processes (McDonald and Macken-Walsh, 2016). When modern testing methods are used to carry out the chemical quality analysis with the required standards it can be beneficial in avoiding any strange results. Chemical quality testing is carried out under strict rules and legislation such as the Antibiotic Testing Regulation, a test that is significant and critical in dairy production. This test is meant to measure the content of penicillin which is an antibiotic in the milk, some consumers are believed to be highly sensitive to penicillin and therefore it can lead to bacterial resistance developing in the population.
Another test which can be carried out in the dairy production industry is the alkaline phosphate test on pasteurised milk which indicates the reliability of pasteurisation as an essential process to make sure the milk is free and safe from pathogens. Pasteurised milk can be contaminated with raw milk and therefore this test is done to ensure its purity. The method is faster and reliable in order to find the enzyme’s presence. Growth in the modern dairy industry has been associated with the introduction of new advanced chemical testing equipment like the MilkoScan FT2 (Foss.co.uk, n.d.); this equipment replaced the Gerber method. It is faster and efficient milk analysing equipment which can detect and test various indicators in milk such as the amount of fat in the milk. It can also calculate the milk density which should range between 1.028g/cm and 1.038g/cm, the protein count and it can measure the freezing point depression (FPD). This indicates the adulteration of milk with water as milk’s normal composition has a freezing point of -0.54°C to -0.59°C (Bilung, 1995). The tests done with such standard equipment can reduce errors and therefore give out more accurate results.
Curdling processes consider acidity and pH when processing dairy products, the best values have to be made in order for curdling process to be undertaken. The correct pH ensures that there are less spoilage and no presence of pathogenic organisms. “Hanna Instruments” is an innovation that tests the acidity and pH in modern dairy industries as it is a microprocessor-based pH meter and automatic titration process. The processor in the equipment assists in eliminating the resulting final colour change indication which can be seen by the human eye during the automatic titration process making it simple to read the pH tester.
Like all other food types, milk and its products can lead to food-borne diseases due to contamination by unsafe chemical contents, the growth of pathogens, degradation of nutrients, and environmental pollution which are known to affect the quality of the milk. Hazards related to microbiology include major dairy product hazards that render milk unsafe for consumption. Chemical components are mostly unintentionally introduced into the dairy products hence making them unsafe. Contamination can take place during the milking process or by animals feeding on poisoned foods or drinking poisonous water. Inadequate control of processing equipment, storage facilities, and the entire environment can cause chemical hazards like teat disinfectants, antibiotics, detergents, pesticides, and melamine among others.
References
- Butler, G. (2011). The effects of dairy management and processing on quality characteristics of milk and dairy products. [online] Sciencedirect.com. Available at: http://www.sciencedirect.com/science/article/pii/S1573521411000212 [Accessed 10 Jan. 2017].
- Bylund, G. (1995). Dairy processing handbook. 1st ed. [Lund, Sweden]: [Tetra Pak Processing Systems AB].
- Effect of storage temperature on the microbiological and physicochemical properties of pasteurized milk. (2013). Annals. Food science and TECHNOLOGY. [online] Available at: http://www.afst.valahia.ro [Accessed 18 Dec. 2016].
- Foss.co.uk. (n.d.). MilkoScan™ FT2 advanced infrared milk analyser for profit in dairy production. [online] Available at: http://www.foss.co.uk/industry-solution/products/milkoscan-ft2/ [Accessed 10 Jan. 2017].
- Mc.Donald, R. and Macken-Walsh, A. (2016). An actor-oriented approach to understanding dairy farming in a liberalised regime: A case study of Ireland’s New Entrants’ Scheme. [online] Sciencedirect.com. Available at: http://www.sciencedirect.com/science/article/pii/S0264837716300114 [Accessed 10 Jan. 2017].
- Merino, L., Örnemark, U. and Toldrá, F. (2016). Analysis of Nitrite and Nitrate in Foods: Overview of Chemical, Regulatory and Analytical Aspects. [online] Advances in food and nutrition research. Available at: https://www.researchgate.net/publication/311703668_Analysis_of_Nitrite_and_Nitrate_in_Foods_Overview_of_Chemical_Regulatory_and_Analytical_Aspects [Accessed 15 Jan. 2017].
- Spellman, D., O’Cuinn, G. and FitzGerald, R. (2005). Physicochemical and sensory characteristics of whey protein hydrolysates generated at different total solids levels. Journal of Dairy Research, [online] 72(2), pp.138-143. Available at: https://www.omicsonline.org/open-access/nutritional-evaluation-and-sensory-characteristics-of-biscuits-flour-supplemented-with-difference-levels-of-whey-protein-concentra-2157-7110-1000546.php?aid=66691 [Accessed 10 Jan. 2017].
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this assignment and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


